
Sulfuric acid and dissolved aluminum in anodizing baths.
Acid-base (or neutralization) titrations depend upon the neutralization of an unknown quantity of acid with a known quantity of base or vice versa. These titrations are used to measure acid concentrations in acid etch tanks, anodizing tanks, and plating tanks, and to measure base concentration in caustic etch tanks. As an example, we will explain the process of testing the sulfuric acid and aluminum concentration in a bath used for MIL-A-8625 Type II anodizing. A similar titration may be used for a MIL-A-8625 Type III anodizing bath; although the concentrations of sulfuric and aluminum are normally different. The following table shows concentrations of acid and aluminum for a typical (Type II) sulfuric anodizing bath. If you prefer ounces and gallons, convert g/L (grams per liter) to oz/gal (ounces per gallon) by dividing by 7.5. The factor of 7.5 is (28.375 grams/ounce)/(3.7854 liters/gallon).
| min | mid | max | |
| Sulfuric acid | |||
| (66°Be H2SO4) | 180 g/L | 190 g/L | 200 g/L |
| Aluminum | |||
| (dissolved) | 4 g/L | 8 g/L | 12 g/L |
1 Pipette a 5.0 mL sample from bath.
2 Dilute with 50 mL of DI water.
3 Add a few drops of methyl orange.
4 Titrate with B mL of 1.0N NaOH from orange to a yellow endpoint.
5 Add a few drops of phenolphthalein indicator.
6 Continue titration to total of A mL of 1.0N NaOH from colorless to pink endpoint.
B mL x Factor = H2SO4
| Sample | Titrant | Factor | |
| H2SO4 (g/L) | 5.0 mL | 1.0N | 9.807 |
| H2SO4 (oz/gal) | 5.0 mL | 1.0N | 1.308 |
[ A mL - B mL ] x Factor = dissolved aluminum
| Sample | Titrant | Factor | |
| Al (g/L) | 5.0 mL | 1.0N | 1.797 |
| Al (oz/gal) | 5.0 mL | 1.0N | 0.240 |
In this analysis, we will determine the concentration of sulfuric acid in a bath sample by titrating with a sodium hydroxide reagent of known normality. We will also determine the amount of dissolved aluminum in the sample by understanding that the solubility of Al(OH)3 varies at different pH levels.
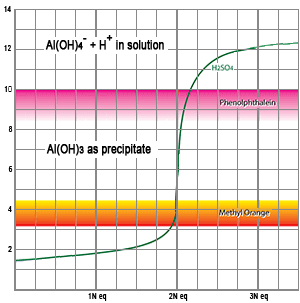
Aluminum hydroxide, Al(OH)3, is soluble in acid (low pH) but insoluble in water (neutral pH). As the pH of a sample increases, the precipitation of OH ions from the bath as solid Al(OH)3 acts as a buffer preventing the pH from rising as more NaOH is added. This buffering effect continues until all of the Al(OH)3 is removed, and then the pH rises very quickly upon further addition of NaOH. Finally, at higher pH, the Al(OH)3 redissolves and drives the pH even higher. If the only chemicals in solution are strong and weak acids, free acid can be measured by titrating to a low pH, and total acid can be measured by titrating to a higher pH. However, when dissolved aluminum is present, the amount of NaOH required to reach a higher pH is not a reliable measure of total acid because of the buffering effect of the Al(OH)3. Nevertheless, the amount of NaOH required to reach a higher pH is proportional to the concentration of dissolved aluminum.
The aluminum hydroxide will remain in solution as long as the pH is low so phenolphthalein cannot be used for this part of the analysis. Methyl orange transitions from orange color to yellow color at pH of 3.6: this color change occurs when the NaOH neutralizes the first H+ from the H2SO4, before there is sufficient NaOH to cause precipitation of the dissolved aluminum or to dissociate the second H+. The first dissociation of the H+ from the H2SO4 has occurred at pH 3.6, so the amount of titrant used in this first part of the analysis can give us a measure of the free acid (or the total amount of H2SO4).
As the sodium hydroxide is added, sodium sulfate and water is formed. The reaction is:
H2SO4 + 2 NaOH —> Na2SO4 + 2 H2O.
From the reaction equation: two moles of NaOH have reacted with one mole of H2SO4, when the solution begins to transition from acid to base (pH of 3.6) so: (98.073 grams) x (1 mole H2SO4/2 moles NaOH) = 49.04 grams of H2SO4 is neutralized by one mole (1 liter of 1.0N) of NaOH. Since the bath sample is 5.0 mL, 49.04/5.0 = 9.807 grams per mL titrant.
At this point in the titration the H2SO4 has been neutralized by the NaOH, but there is insufficient NaOH in solution to precipitate the aluminum hydroxide. Phenolphthalein changes from clear to a pink color at pH of 8.6. This pH indicates near completion of the reaction when the solution becomes highly alkaline due to an abundance of free OH- ions.
As the sodium hydroxide continues to be added, the pH increases. Since aluminum hydroxide is soluble in acid but insoluble in neutral solutions, it precipitates and removes OH ions, thereby acting as a buffer: the NaOH increases the pH, but the precipitating Al(OH)3 buffers the change as long as there is Al(OH)3 remaining in solution. For every 3 molecules of NaOH, one molecule of Al(OH)3 is formed. The reaction is:
Al2(SO4)3 + 6 NaOH —> 3Na2SO4 + 2Al(OH)3
From the reaction equation: six moles of NaOH have reacted with two moles of Al when the solution begins to transition from neutral to base (pH of 8.6) so: 2 x (26.982 grams) x (2 moles Al/6 moles NaOH) = 8.985 grams of Al reacts with one mole (1 liter of 1.0N) of NaOH. Since the bath sample is 5.0 mL, 8.985/5.0 = 1.797 grams per mL of additional titrant, where the additional titrant is A mL - B mL.
This method analyzes acid and aluminum using one bath sample; however, there is a more accurate analysis that is normally used by aerospace suppliers. See Anodize Method 2 for details.
Electro-Plating, A Survey of Modern Practice
Samuel Field and A. Dudley Weill
Pitman Publishing, 1951 (Pg 450)
Titrating Sulfuric Acid Anodizing Baths
Larry Chesterfield
Anodizing Technologies
Products Finishing, 2012